The following is an excerpt from a new research report from ALM Intelligence on consulting services in the Healthcare industry. For more information, please visit consulting.almintel.com
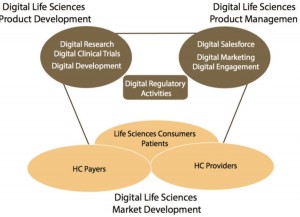
Innovation to Define the Next Digital LS Market Opportunities
To continue reading, become an ALM digital reader
Benefits include:
- Authoritative and broad coverage of the business of consulting
- Industry-leading awards programs like Best Firms to Work For, Global Leades and Rising Stars
- An informative newsletter that goes into the trends shaping the industry
- Critical coverage of the employee benefits and financial advisory markets on our other ALM sites, BenefitsPRO and ThinkAdvisor
Already have an account? Sign In Now